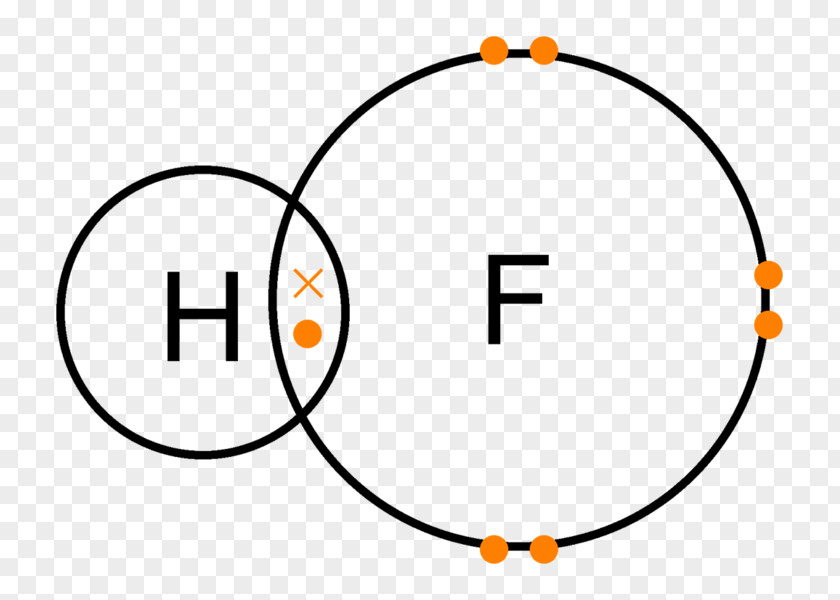
Why Hydrogen Fluoride Is A Liquid At Room Temperature . hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. What does it look like? anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. As the lightest of the hydrogen halides, it has. the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. hydrogen fluoride is a gas at room temperature. Its solution in water is known as hydrofluoric acid. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. hydrogen fluoride is a liquid: Hydrogen fluoride has an abnormally high boiling. When mixed with water it is called ‘hydrofluoric acid’.
from pnghero.com
hydrogen fluoride is a liquid: anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Hydrogen fluoride has an abnormally high boiling. hydrogen fluoride is a gas at room temperature. Its solution in water is known as hydrofluoric acid. When mixed with water it is called ‘hydrofluoric acid’. hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. What does it look like? pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c.
Hydrogen Fluoride Lewis Structure Covalent Bond Chemical PNG Image PNGHERO
Why Hydrogen Fluoride Is A Liquid At Room Temperature Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. hydrogen fluoride is a liquid: hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride has an abnormally high boiling. Its solution in water is known as hydrofluoric acid. hydrogen fluoride is a gas at room temperature. the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. As the lightest of the hydrogen halides, it has. What does it look like? anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid.
From www.chegg.com
Solved Fluorine gas and water vapor react to form hydrogen Why Hydrogen Fluoride Is A Liquid At Room Temperature Hydrogen fluoride has an abnormally high boiling. hydrogen fluoride is a liquid: the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. When mixed with water it is called ‘hydrofluoric acid’. anhydrous hydrogen fluoride is a gas at an. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.boreal-laser.com
Hydrogen Fluoride Gas Detection Boreal Laser Why Hydrogen Fluoride Is A Liquid At Room Temperature the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. As the lightest of the hydrogen halides, it has. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. When mixed with water it is called ‘hydrofluoric acid’. pure hydrogen fluoride is a colourless, fuming. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.alamy.com
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the Why Hydrogen Fluoride Is A Liquid At Room Temperature Its solution in water is known as hydrofluoric acid. hydrogen fluoride is a liquid: hydrogen fluoride is a gas at room temperature. Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. As the lightest of the hydrogen halides, it has. pure hydrogen fluoride is a colourless, fuming liquid that boils at. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.wbir.com
What is hydrogen fluoride? Why Hydrogen Fluoride Is A Liquid At Room Temperature hydrogen fluoride is a gas at room temperature. Its solution in water is known as hydrofluoric acid. Hydrogen fluoride has an abnormally high boiling. hydrogen fluoride is a liquid: the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. When. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From elecschem.com
The Structure and Properties of a Hydrogen Fluoride Molecule Exploring the Diagram Why Hydrogen Fluoride Is A Liquid At Room Temperature As the lightest of the hydrogen halides, it has. hydrogen fluoride is a gas at room temperature. Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. Hydrogen fluoride has an abnormally high boiling. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. What does it look like? When mixed. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.dreamstime.com
Hydrofluoric Acid, Hydrofluoride, HF Molecule. it is Solution of Hydrogen Fluoride in Water Why Hydrogen Fluoride Is A Liquid At Room Temperature Hydrogen fluoride has an abnormally high boiling. When mixed with water it is called ‘hydrofluoric acid’. the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. What does it look like? hydrogen fluoride is a gas at room temperature. Its. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides. Explain why hydrogen Why Hydrogen Fluoride Is A Liquid At Room Temperature As the lightest of the hydrogen halides, it has. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride has an abnormally high boiling. hydrogen fluoride is a liquid: Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. hydrogen fluoride is a gas at room temperature. What does it look like?. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.researchgate.net
Brillouin light scattering spectra of hydrogen fluoride at the... Download Scientific Diagram Why Hydrogen Fluoride Is A Liquid At Room Temperature the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. When mixed with water it is called ‘hydrofluoric acid’. hydrogen fluoride is a liquid: As the lightest of the hydrogen halides, it has. pure hydrogen fluoride is a colourless, fuming. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.science.org
Roomtemperature cycling of metal fluoride electrodes Liquid electrolytes for highenergy Why Hydrogen Fluoride Is A Liquid At Room Temperature hydrogen fluoride is a liquid: What does it look like? the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. hydrogen fluoride is a gas at room temperature. Its solution in water is known as hydrofluoric acid. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.bigstockphoto.com
Hydrogen Fluoride Image & Photo (Free Trial) Bigstock Why Hydrogen Fluoride Is A Liquid At Room Temperature anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. Hydrogen fluoride has an abnormally high boiling. What does it look like? the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.differencebetween.com
Difference Between Hydrogen Fluoride and Hydrofluoric Acid Compare the Difference Between Why Hydrogen Fluoride Is A Liquid At Room Temperature the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. When mixed with water it is called ‘hydrofluoric acid’. As the lightest of the hydrogen halides, it has. Its solution in water is known as hydrofluoric. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides. Explain why hydrogen Why Hydrogen Fluoride Is A Liquid At Room Temperature hydrogen fluoride is a gas at room temperature. As the lightest of the hydrogen halides, it has. What does it look like? Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. pure hydrogen fluoride. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.numerade.com
SOLVED MEEN 2211 Materials Engineering Fall 2019/20 Q5 Briefly cite the main differences Why Hydrogen Fluoride Is A Liquid At Room Temperature As the lightest of the hydrogen halides, it has. What does it look like? When mixed with water it is called ‘hydrofluoric acid’. hydrogen fluoride is a liquid: Its solution in water is known as hydrofluoric acid. the hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. anhydrous hydrogen fluoride is a. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.researchgate.net
(PDF) Reaction of Hydrogen Fluoride Gas at High Temperatures with Silicon Oxide Film and Silicon Why Hydrogen Fluoride Is A Liquid At Room Temperature Due to the high electronegativity of the fluorine atom, there is strong intermolecular hydrogen bonding. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. Hydrogen fluoride has an abnormally high boiling. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Its solution in water is known as. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From mavink.com
Hydrogen Fluoride Diagram Why Hydrogen Fluoride Is A Liquid At Room Temperature What does it look like? hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. When mixed with water it is called ‘hydrofluoric acid’. Hydrogen fluoride has an abnormally high boiling. Its solution in water is known as hydrofluoric acid. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. anhydrous hydrogen fluoride is. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.researchgate.net
Brillouin light scattering spectra of hydrogen fluoride at the... Download Scientific Diagram Why Hydrogen Fluoride Is A Liquid At Room Temperature When mixed with water it is called ‘hydrofluoric acid’. As the lightest of the hydrogen halides, it has. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Hydrogen fluoride has an abnormally high boiling. pure hydrogen fluoride is a colourless, fuming liquid that boils at 19.5°c. the hydrogen halides. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.researchgate.net
Phase diagram of hydrogen. The pressure path at room temperature in the... Download Scientific Why Hydrogen Fluoride Is A Liquid At Room Temperature hydrogen fluoride (hf) is an extremely toxic, corrosive gas and liquid. hydrogen fluoride is a gas at room temperature. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. When mixed with water it is called ‘hydrofluoric acid’. the hydrogen halides are colourless gases at room temperature, producing steamy. Why Hydrogen Fluoride Is A Liquid At Room Temperature.
From www.alamy.com
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the Why Hydrogen Fluoride Is A Liquid At Room Temperature As the lightest of the hydrogen halides, it has. hydrogen fluoride is a liquid: Hydrogen fluoride has an abnormally high boiling. anhydrous hydrogen fluoride is a gas at an ordinary temperature, which condenses to a colourless fuming liquid. Its solution in water is known as hydrofluoric acid. pure hydrogen fluoride is a colourless, fuming liquid that boils. Why Hydrogen Fluoride Is A Liquid At Room Temperature.